During a recent Transparency & Material Health in Practice panel discussion, Colden’s Catherine Bobenhausen shared a concept that is fundamental to the work of industrial hygiene, the hierarchy of controls, as illustrated below:
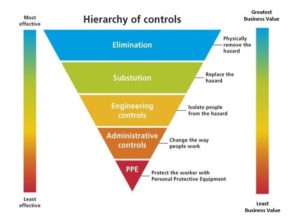
This triggered a conversation between Catherine and Wendy Vittori, Executive Director of the Health Product Declaration Collaborative.
WV: That Hierarchy of Controls concept you shared is a wonderful way to envision the approaches taken by industrial hygienists to improve the safety of the process at the plant floor level. What I am most interested in for architects and other designers is how to use this as an analogy for how they can think about improving the safety of the building they are designing, when thinking about the use of building products whose chemical contents have been disclosed. While eliminating products using “Red List” chemicals is what a designer might want to achieve – that is often the most difficult, highest order request and not feasible given the range of product choices they have. So, the analogy would be how could we adapt this hierarchy to inform product decision-makers about available, and best options, to maximize the safety and health characteristics of their product selections. Depending upon the product, incremental steps are used to reach that goal. Using this hierarchy, manufacturers could inform product decision-makers, using transparency reports, on where they are now in managing chemicals of concern in their products, and inform their customers about the steps they are taking to reformulate their products to introduce improved ingredients, reducing and eliminating chemicals of concern. This type of reporting would enable the decision-maker to better calibrate the reported chemical and health hazard information the manufacturer is providing.
CB: Yes, I agree. And first, it is critical to have a really fine-tuned understanding of what the material is – until recently, architects never thought to ask what was in the building materials they specified. Now, they are starting to ask for Health Product Declarations and with those in hand, they are demanding more of the manufacturers – in many cases, their reaction to the disclosed information is to eliminate all disclosed chemicals that are present at any concentration that are listed as carcinogens, mutagens, reproductive or developmental toxicants, and those associated with endocrine activity and/or are persistent, bioaccumulative or toxic. As non-chemists, they may expect that this is a simple swap-out that manufacturers can easily and quickly accomplish.
They can be put off when the response from the manufacturer is that their preferred products can’t be so rapidly reformulated. While it is possible that a manufacturer is responding this way because they don’t have plans to reformulate – or don’t wish to – on the plus side, I’ve seen owners and architects and manufacturers working well together with best intentions and with a very nuanced and sophisticated understanding that change takes time and timely progress is possible. The challenge from the design professional is tackled with urgency and robust planning, scheduling, testing and roll out. A key learning from these experiences is that an open, collaborative innovation approach – with owners and design professionals collaborating directly with manufacturers – is a powerful method for transforming products to address safety and health concerns.
WV: Yes. And let’s not underestimate the power of the Ask – Asking for product transparency reporting. The very fact that design professionals are asking has been a strong motivator, leading to a tremendous amount of innovation as salespeople have reported back to their companies what the architects and designers need. From the manufacturers who are part of HPDC’s membership, I understand that this has led many to initiate research into new formulations to achieve equal results and take action to make product improvements a reality. In general, these efforts become forerunners in their overall product design process, which begins to incorporate an upstream consideration of chemical health characteristics from the start of the product design process. With increased demand, innovations are rolled out across a manufacturer’s portfolio of products.
When design professionals provide clear incentives for improvement – beginning with basic transparency reporting – manufacturers can see that the market demand for these actions is real. Rewarding manufacturers who get onto a path of incremental optimization is key. I like what John Amatruda of Vidaris is doing with his Finish Line approach, which builds a rational, tiered process of product selection and recognition. It uses a process of incremental optimization roughly analogous to the Hierarchy of Controls approach.
CB: I agree with you. I know organizations that harmonize with the Health Product Declaration Collaborative such as Cradle to Cradle, the U.S. Green Building Council and the International Living Future Institute build in systems for recognizing gradual improvement in product lines. This is where the market is headed, and it is a very good thing to see the type of progress that has been driven by interaction of designers with manufacturers. First, transparency meaning the manufacturers disclose what is in the products they formulate, which leads to a frank discussion on next steps to improve the health/safety performance of these products while maintaining functionality, performance and cost. This is a tall order, but a very positive transformation. It is what the industrial hygiene profession aspires to, eliminating hazard as the top priority, and encouraging and incentivizing incremental progress toward that end, to speed progress toward the ultimate goal of transformation to safer products overall.

